

RADIESSE® Treatment Informed Consent
I, _______________________________ understand that I will be injected with
RADIESSE dermal filler in the following areas:
____________________________________ .
RADIESSE dermal filler is a resorbable implant product approved by the United
States Food and Drug Administration for the correction
of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Risks and complications that may be associated with RADIESSE dermal filler and
the implant procedure include, but are not limited to:
1. Facial Bruising, Redness, Swelling, Itching and Pain: I understand that there
is a risk of bruising, redness, swelling, itching and pain
associated with the procedure. These symptoms are usually mild and last less
than a week but can last longer. Patients who are using
medications that can prolong bleeding, such as aspirin, warfarin, or certain
vitamins and supplements, may experience increased bruising
or bleeding at the injection site.
2. Nodules, and palpable material: I understand that there is a risk that small
lumps may form under my skin due to the RADIESSE
filler material collecting in one area. I also understand that I may be able to
feel the RADIESSE filler material in the area where the
material has been injected. Any foreign material injected into the body may
create the possibility of swelling or other local reactions to a
filler material.
3. Migration: I understand that the RADIESSE dermal filler, as with any filler
material, may move from the place where it was injected.
4. Infection: As with all transcutaneous procedures, I understand that injection
of any filler material carries the risk of infection.
5. Allergic Reactions: I understand that RADIESSE dermal filler should not be
used in patients with severe allergies, a history of
anaphylaxis, or history or presence of multiple severe allergies or
hypersensitivity to any of the ingredients in RADIESSE filler.
6. Keloids/Scarring: I understand that the safety of RADIESSE dermal filler in
patients with known susceptibility to keloid formation or
hypertrophic scarring has not been studied.
7. Accidental Injection into a Blood Vessel: I understand that RADIESSE dermal
filler can be accidentally injected into a blood vessel,
which may block the blood vessel and cause local tissue damage, or potentially
even a heart attack or stroke.
8. Radio-opacity: I understand that RADIESSE dermal filler is radio-opaque and
is visible on CT Scans and may be visible in x-rays.
9. Duration of Effect: I understand that the outcome of treatment with RADIESSE
dermal filler will vary among patients. In some
instances, additional treatments may be necessary to achieve the desired
outcome.
No studies of interactions of RADIESSE dermal filler with drugs or other
substances or implants have been conducted.
This above list is not meant to be inclusive of all possible risks associated
with RADIESSE dermal filler or dermal fillers in general, as
there are both known and unknown side effects and complications associated with
any medication or dermal filler injection procedure. I
understand that medical attention may be required to resolve complications
associated with my injection.
I understand that I should minimize exposure of the treated area to the sun or
heat for approximately 24 hours after treatment or until any
initial swelling or redness goes away.
The safety of RADIESSE dermal filler for use during pregnancy or in
breastfeeding women has not been established.
I have discussed the potential risks and benefits of RADIESSE dermal filler with
my doctor. I understand that there is no guarantee of any
particular results of any treatment.
I understand and agree that all services rendered will be charged directly to
me, and I am personally responsible for payment. I further
agree, in the event of non-payment, to bear the cost of collection, and/or court
costs and reasonable legal fees, should they be required. By
signing below, I acknowledge that I have read the foregoing informed consent,
have had the opportunity to discuss any questions that I
have with my doctor to my satisfaction, and consent to the treatment described
above with its associated risks. I understand that I have the
right not to consent to this treatment and that my consent is voluntary. I
hereby release the doctor, the person performing the RADIESSE
filler injection and the facility from liability associated with this procedure.
________________________________________________________________________
Patient Signature Date
This consent form was provided by Radiesse with some minor modifications. Disclaimer - use at your own risk. We offer fillers at our office (Surgical Artistry) Telephone (Modesto, CA) 209-551-1888. We are located in Modesto, CA (2336 Sylvan Ave. Suite C, Modesto, CA 95355). We offer this webpage as a resource for our patients and a source of educational material for our future patients. This webpage was written in February 2010 - things may have changed since.
Dr. Wu and Dr. Lee operating together on a cosmetic surgery
at DMC (Doctor's Medical Center), Modesto
Our Surgeon Injectors:
At Surgical Artistry, injections are provided by Dr. Calvin Lee and Dr. Tammy Wu, both of them are board certified surgeons. Dr. Calvin Lee is a general surgeon and Dr. Tammy Wu is a plastic surgeon. Both work together at Surgical Artistry in Modesto, CA.
We treat injections as surgical procedures - with care, dexterity, and sterility.
For more details regarding the surgeon injectors of these fillers, you can visit our Modesto Botox website which displays a brief bio of Dr. Calvin Lee and Dr. Tammy Wu (here).
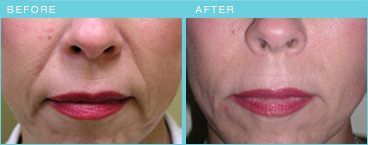
Picture from Radiesse company, not actual plastic surgery
patient of ours.
For more information regarding Radiesse wrinkle fillers (the source of this post treatment checklist), please visit their website

(209) 551-1888
Located in
Modesto, CA
Services in Plastic Surgery, Veins, Acupuncture, and General Surgery
email clee [at] surgerytoday.com
[return to Home page - Plastic Surgery Modesto]